PREDICT, un programa epidemiológico multinacional bien financiado patrocinado por la Agencia de los Estados Unidos para el Desarrollo Internacional (USAID), hasta que finalizó en 2019, el programa buscaba patógenos en animales y humanos para detectar nuevas amenazas pandémicas. La Dra. Supaporn es uno de los cazadores de virus más exitosos del mundo. Es conocida por su trabajo de seguimiento del virus Nipah, un patógeno transmitido por murciélagos que es menos contagioso que el SARS-CoV-2 pero más letal para los humanos. Ha encontrado coronavirus de murciélago relacionados con el SARS-CoV, que desencadenó la epidemia del síndrome respiratorio agudo repentino (SARS) hace casi dos décadas, y el virus detrás del síndrome respiratorio de Oriente Medio (MERS). A principios de marzo, se reunió con investigadores de un nuevo proyecto de 5 años de $125 millones lanzado el año pasado por USAID llamado Descubrimiento y exploración de patógenos emergentes: zoonosis virales (DEEP VZN).
In rural Thailand, an elephant sitting in the road is not a charming sight. The massive beasts have a penchant for ripping off bumpers, tusking doors, and sitting on hoods. So in January, when an elephant loomed on the pavement ahead, a van carrying a team of bat researchers on a road 200 kilometers southeast of Bangkok stopped abruptly. As the animal loped toward the van, ears flapping and trunk swinging, the driver slowly backed up. At last, the elephant lumbered back into the other lane and the driver crept past. “That was wild!” a member of the team said.
Its leader, Supaporn Wacharapluesadee, who stands out as mild mannered in a famously mild-mannered culture, fell off her seat laughing with relief. She is used to much smaller, but more consequential, menaces. Within hours, she and her team planned to be in Thailand’s Khao Ang Rue Nai Wildlife Sanctuary examining animals for dangerous viruses that might spill over into humans—or already have.
Supaporn is one of the world’s most accomplished virus hunters. She is known for her work tracking Nipah virus, a batborne pathogen that is less contagious than SARS-CoV-2 but more deadly to humans. She has found bat coronaviruses related to both SARS-CoV, which triggered the epidemic of sudden acute respiratory syndrome (SARS) nearly 2 decades ago, and the virus behind Middle East respiratory syndrome (MERS). And her quest has gained new importance during the COVID-19 pandemic, which likely originated when a bat coronavirus evolved into SARS-CoV-2 and crossed over into humans, perhaps through an intermediate host animal.
She was the first researcher to sequence SARS-CoV-2 outside China—not in an animal, but in an airline passenger—and she is on the trail of its wild relatives. From her base at Chulalongkorn University in Bangkok, Supaporn has made many forays like the one delayed by the elephant. Those outings added precious data points in the hunt for SARS-CoV-2’s origin as she identified bat coronaviruses on the virus’ family tree—some of which may be its closest relatives yet found.

The 52-year-old scientist’s career blossomed over the past decade after she joined PREDICT, a multicountry, well-funded epidemiological program sponsored by the U.S. Agency for International Development (USAID). Until it ended in 2019, the program looked for pathogens in animals and humans to spot new pandemic threats. The World Health Organization in fall 2021 named her a member of its new Scientific Advisory Group for the Origins of Novel Pathogens.
“She’s fabulous,” says Dennis Carroll, a tropical disease specialist who started PREDICT. “She’s demonstrated over the years a really innovative mind in terms of the fieldwork she does, and she’s extremely practical, doing really high-quality lab work.”
PREDICT’s principal investigator, epidemiologist Jonna Mazet of the University of California, Davis, also admires how Supaporn has made her way in a male-dominated field. “She’s had to fight for what she got, which is especially impressive in a country like Thailand, where the women are not as supported as they are here in the U.S.”
Yet some scientists—including Supaporn’s former boss, Thiravat Hemachudha—question whether the type of arduous wild animal surveillance she did on that elephant-interrupted January trip truly makes humans safer. “I don’t think it’s that valuable, and it may be dangerous,” says Thiravat, a neurologist who last year had a complicated falling out with Supaporn that has left her without lab equipment and staff.
Thiravat and other scientists contend that the most efficient way to head off new pandemics is to more aggressively test sick livestock and other animals in contact with people, as well as people with unexplained illnesses, and intensify surveillance of people who often interact with animals harboring dangerous pathogens. “Our motto is: Minimize budget and maximize benefit,” Thiravat says.
Supaporn, who hopes to take part in two new viral sleuthing efforts designed to derail spillovers, including a proposed multibillion-dollar Global Virome Project (GVP), says critics are presenting a false choice. To understand viral threats, she says, wildlife surveillance is as important as testing people and livestock. “If we don’t do anything, we will not know anything,” she says. She and other pathogen hunters say if earlier findings from wild animals had been taken more seriously, “coronavirus” would not have become a common word in every spoken language.
SUPAPORN’S PARENTS MADE fittings for jewelry, and as a child she thought she would become an artist like her brother. But as a teen she realized her talent lay in science. She earned an undergraduate degree in medical technology and spent 10 years working in several diagnostic labs. “When I was young, I was not a communicative person, so working in the lab, there was no need to talk to anyone,” Supaporn says. “I thought being a technician was the best job for me.”
But when a supervisor hired an outside company to solve an assay problem that she knew how to fix herself, she decided her tech days had ended. “I thought, ‘I can do more than that.’”
In graduate school, she studied with Thiravat, who treated people infected with rabies, mainly through dog bites. A related virus that infects Australian bats also causes a rabieslike disease in humans, so she and Thiravat decided in 2002 to start sampling bats in Thailand. The bats carried antibodies to that second virus, indicating its presence in Thailand as well. At the government’s behest, the researchers also began sampling bats and other animals for Nipah virus, which emerged in Malaysian pigs and their farmers in 1998, killing up to 75% of infected humans.
All in the family
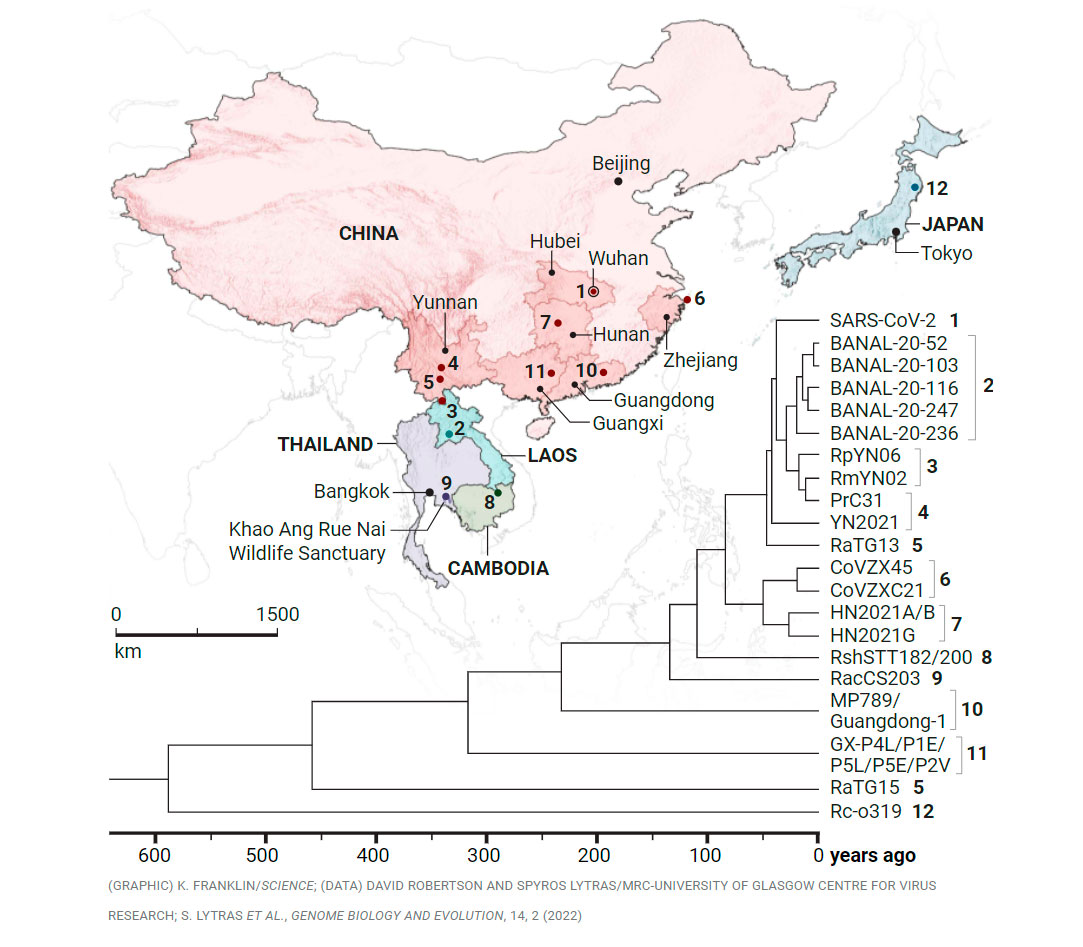
Coronaviruses related to SARS-CoV-2 have turned up in Rhinolophus bats roosting all across Asia. Differences between viral sequences have enabled researchers to build a family tree and estimate that the closest relatives shared a common ancestor with the pandemic virus a decade ago. Supaporn Wacharapluesadee’s team found a virus in Thailand (No. 9) that shared a relative about 140 years ago and has identified but not yet published closer relatives.
Supaporn, Thiravat, and colleagues repeatedly found antibodies to Nipah in Pteropus, or flying foxes, the world’s largest bats with a 1.5-meter wingspan. Eventually, the team isolated the virus itself from a bat. To dispel folklore about a popular aphrodisiac in Thailand and other Asian countries, they published a paper in Clinical Infectious Diseases in 2006 with a startling title: “Drinking Bat Blood May Be Hazardous to Your Health.”
To prevent Nipah spillovers, for 2 decades Supaporn has tested humans and pigs in villages near Wat Luang Phrommawat, a 400-year-old temple with a grove of trees where some 10,000 flying foxes roost. She has never found Nipah virus or its immunological footprints in humans or pigs, but Supaporn says the work led the locals to discard fruit that was partially eaten, possibly by the bats.
“I have a responsibility to the community to do education about this risk,” she says.
The Nipah studies caught the attention of scientists at the EcoHealth Alliance, a conservation-oriented nonprofit in New York City that was part of PREDICT, and in 2009 it subcontracted with Supaporn to do wildlife surveillance in Thailand. Peter Daszak, who heads EcoHealth, notes that few researchers in the countries where pandemics tend to originate do such work.
“Supaporn’s one of those who gets it,” says Daszak, who has been scrutinized because of the possibility—dismissed by many scientists as pure speculation—that SARS-CoV-2 leaked from a lab EcoHealth collaborated with at the Wuhan Institute of Virology in China. “And it’s not easy for someone to develop their own pathway like she has.”
Since then, Supaporn has done multiple studies with EcoHealth and PREDICT. She showed that bat guano used as fertilizer by Thai farmers was contaminated with a coronavirus related to the cause of MERS, and she ranked the spillover potential of different animal viruses. Even before the pandemic, she had described 63 coronavirus sequences detected in 13 species of Thai bats she sampled.
Shi Zhengli, who runs the Wuhan lab and also has come under attack by lableak proponents, has collaborated with Supaporn and says they often swap ideas. “Tropical Asia is a hot spot of wildlife-borne emerging infectious diseases,” Shi says, “so her job is very important for disease prevention and precaution in the region.”
THE KINDS OF THREATS Supaporn had been tracking became catastrophically real at the beginning of 2020. On 8 January, a passenger arriving from Wuhan at Bangkok’s international airport registered hot on thermal scanning equipment. An ear check showed her temperature was 38.1°C. Rome Buathong, a field epidemiologist for the Thai Ministry of Public Health who had set up the scanners 5 days earlier when news arrived about the outbreak in Wuhan, promptly sent the woman to the hospital. All viral tests were negative, so Rome contacted Supaporn, who had worked with him years earlier to screen air passengers for Ebola and Zika viruses.
On 9 January—the day before Chinese researchers first publicly reported SARS-CoV-2’s genome—Supaporn discovered the genetic signature of a novel virus in that Wuhan visitor, becoming the first scientist outside China to do so. A database search showed the new virus was closest to a coronavirus in Chinese bats that Daszak and Shi had reported in 2017. “Ten years ago, no one thought bats were important—we thought only about influenza,” Rome says. “But Supaporn was very keen to do a lot with bats. Who knew?”

The COVID-19 pandemic in early 2020 halted field research globally, but Supaporn, with funding from the U.S. Department of Defense’s Biological Threat Reduction Program, managed that June to send a team to a large cave in western Thailand that is home to a few million bats. The endeavor was part of a general pathogen surveillance effort, but the group hoped to find a clue to SARS-CoV-2’s origin by sampling Rhinolophus bats, also known as horseshoe bats for the shape of their noses. The genus, comprising more than 100 species, is the main host for SARS-related coronaviruses.
Horseshoe bats live in small colonies that are often hard to find, and the cave didn’t yield any. But in a water pipe draining a reservoir that’s part of the Khao Ang Rue Nai Wildlife Sanctuary, Supaporn’s team trapped 100 Rhinolophus acuminatus. Rectal swabs from 13 tested positive for coronaviruses, including one described in Nature Communications on 9 February 2021. Dubbed RacCS203, the virus was 91.5% identical in genetic sequence with SARS-CoV-2. That similarity implied a common ancestor from about 140 years ago, according to an analysis led by evolutionary biologists David Robertson and Spyros Lytras of the MRC-University of Glasgow Centre for Virus Research, published online on 8 February in Genome Biology and Evolution.
Other researchers found bat coronaviruses related to SARS-CoV-2 in China, Laos, Vietnam, Cambodia, and Japan. One virus from a colony in limestone caves in Laos was 96.8% similar in sequence to the human virus—perhaps a decade removed. Even it is too distant to offer anything more than crumbs on the evolutionary path that led to the pandemic virus. But Robertson is convinced that Asia’s bats harbor far closer relatives to SARS-CoV-2. “There’s definitely something that’s not been sampled,” he says.

On the trip this January, Supaporn returned to the sanctuary in search of closer matches. RacCS203, unlike the virus from Laos, does not infect by binding to the human cellular receptor favored by SARS-CoV-2. But antibodies in the blood of bats in the sanctuary powerfully neutralized the pandemic virus, suggesting they may have been infected with a coronavirus that uses that receptor, too.
Some researchers think the bat virus hunt will do little to clarify the pandemic’s origin. A distant bat precursor to SARS-CoV-2 might have spread long ago to an intermediate host—perhaps a rat, civet cat, raccoon dog, or pangolin, all known to host bat viruses—and evolved there for years before infecting humans. But Supaporn is betting she’ll find revealing clues in bats. “It would be good to fill in the gaps of the origin story in Southeast Asia because in Thailand alone there are 23 Rhinolophus species,” she says.
Filling in the gaps is a painstakingly slow, expensive, risky, and often hugely unpleasant process. “You’re looking for something rare, and you need a ton of samples to pick up the rare thing,” Mazet says.
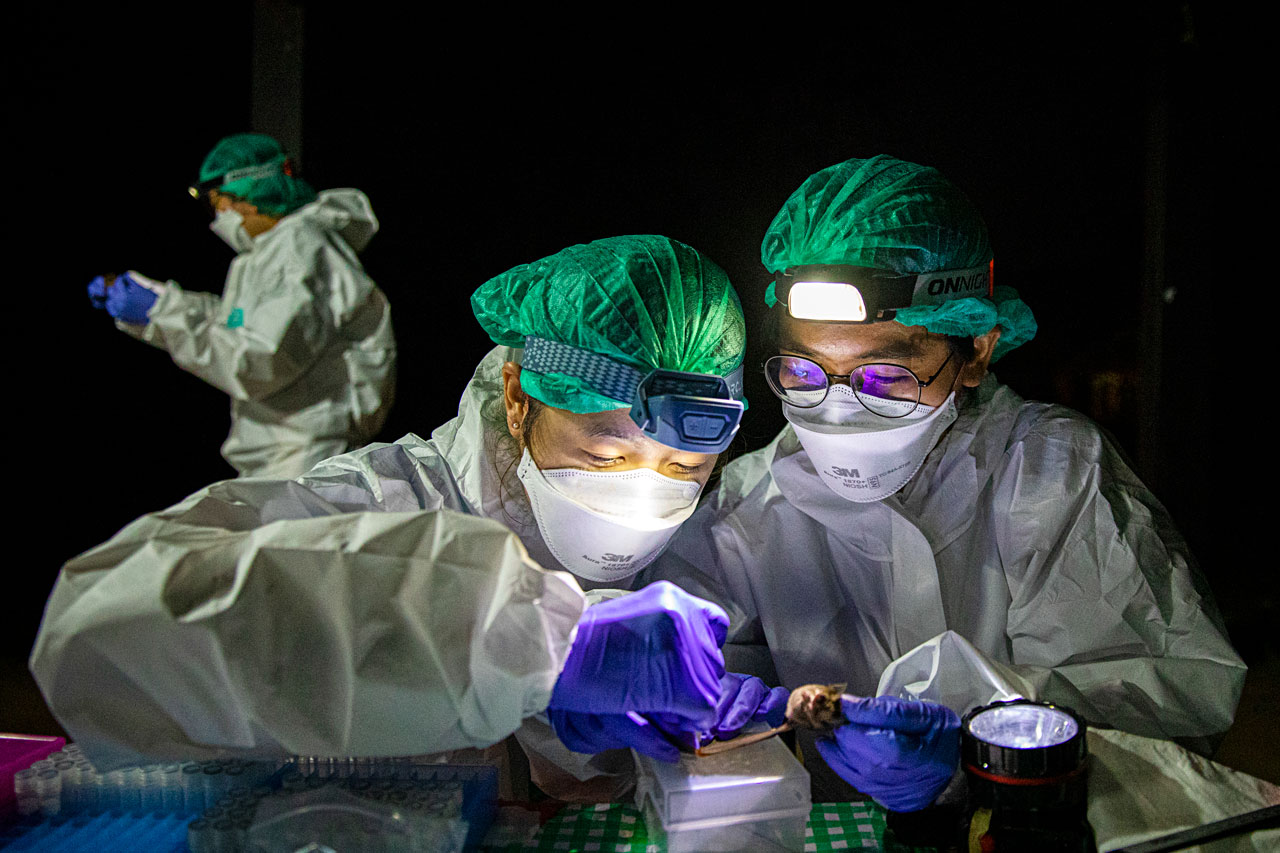
BY THE TIME SUPAPORN’S van passed the elephant and joined the rest of the team at the field site, it was after 4 p.m. With military efficiency, the team—two dozen grad students, ecologists, and veterinarians—set up a lab on the ground floor of an abandoned traditional Thai house on stilts. The first order of business, ironically, was to protect the bats from human viruses, including SARS-CoV-2: Everyone had nasal swabs, which came back negative.
Next, team members put on hairnets, polyethylene coveralls, nitrile gloves, and N95 masks to protect themselves. The temperature was 32°C. Sweat soon soaked every bit of fabric under the zipped-up suits.
A half-dozen men, who also donned rubber boots and mining headlamps, left the lab and went down an adjacent road to the water pipe, home to a few hundred R. acuminatus. The group scuttled down a ladder to the pipe’s opening. Butterfly nets in hand, they hunched into the tunnel, where the stench of bat feces, urine, and wet fur parked in the nose. A mesh placed over the pipe’s opening caught any bats trying to leave the roost.

Emerging from the pipe, the men untangled the mouse-size bats from their nets, a delicate process given the tangle of spiny wings in the mesh and the animals’ ice pick teeth. Each bat went into its own cloth bag. Supaporn did not take part in the procedure. “I’m not good at it,” she says, noting that she has been bitten several times.
The next day, the team trapped another 50 Rhinolophus from the water pipe. The group also captured 50 bats of another species, Hipposideros, from beneath a botanical museum at the wildlife sanctuary, so they could be tested to see whether any coronaviruses had jumped from the Rhinolophus roosting nearby. After each trapping, Supaporn’s team took the animals back to the field station for measurements and tissue samples, aiming to free the bats as quickly as possible to minimize trauma and harm.
“They’re one of the more efficient teams I’ve worked with,” says Kevin Olival, an ecologist at EcoHealth. “In many other countries, it would take 5, 6, 7 days to get that many bats.”
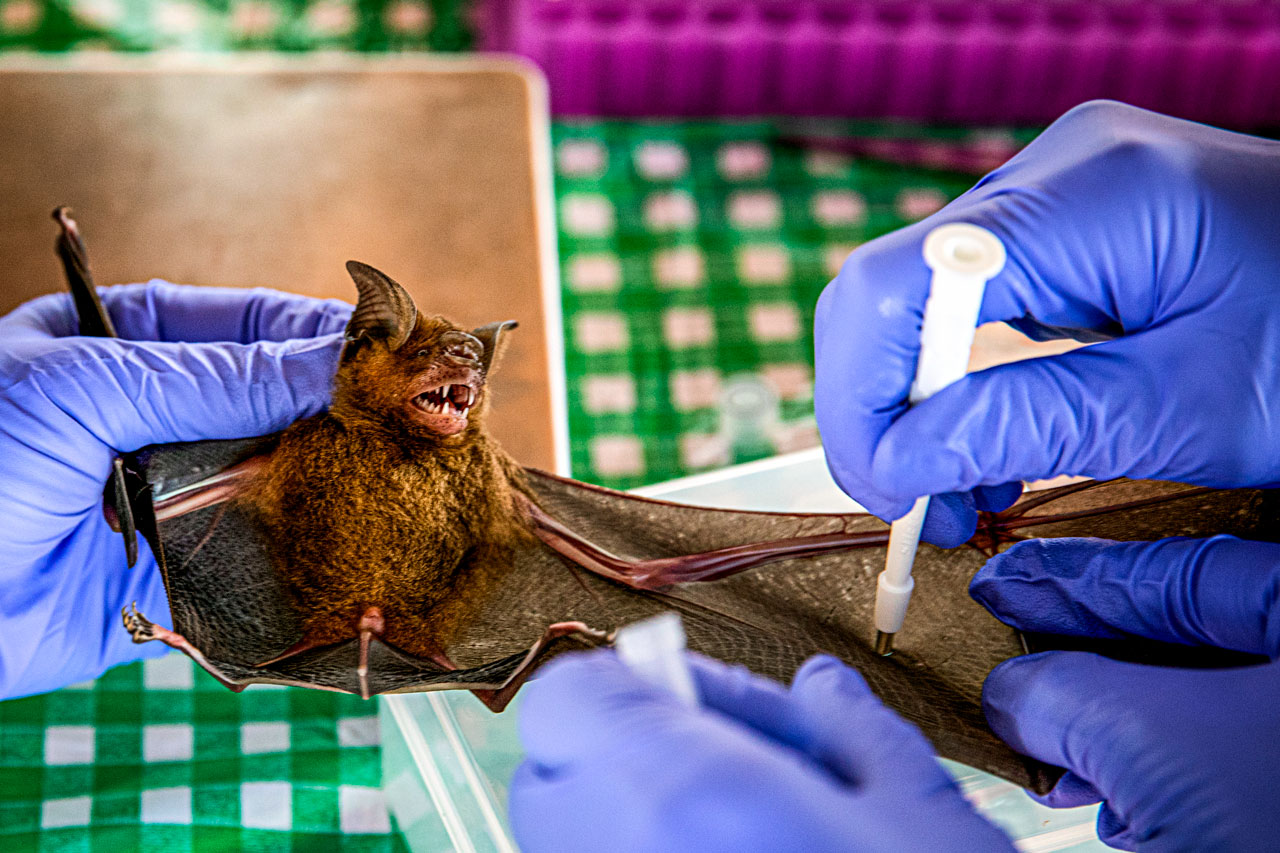
The field station resembled a production line. At the first cluster of tables, team members weighed each bat, measured head and ear size with a caliper, shone a light through the wing to estimate age from bone joint size, measured wingspan, and tweezed off parasites, saving them in tiny tubes for a separate study. Station two swabbed the anus and mouth, hole punched tissue from a wing, aspirated blood from a capillary, and then brushed red nail polish on toes so no released bat would be sampled twice.
The swabs were later tested for viral genetic material and the wing tissue for DNA confirmation of the species. Supaporn and her collaborators in other countries will test the blood for antibodies against a wide range of paramyxoviruses, influenza viruses, filoviruses, and coronaviruses.
Supaporn worked at a third station, centrifuging bat blood to separate the plasma. She took an occasional breather to pluck a cloth bag from the end of the production line, smiling broadly each time she nudged out a bat with red nail polish and watched it fly off toward home.
Data from the January trapping, to Supaporn’s surprise, indicated coronaviruses unrelated to the SARS family in the Hipposideros but none in the Rhinolophus. Antibody analyses are still underway, and she suspects many Rhinolophus will test positive for past infections with SARS-related viruses.
Another foray, a March 2021 expedition to a cave west of Bangkok, yielded two new SARS-CoV-2–related coronaviruses in a species of Rhinolophus called R. pusillus. Supaporn analyzed them with Linfa Wang, a specialist in emerging infectious diseases at Duke-NUS Medical School in Singapore who in 2013 co-wrote a paper with Shi and Daszak describing the first bat coronavirus linked to SARS-CoV. Wang says he and Supaporn plan to report that in some parts of the viral surface protein that docks onto animal cells, the new viruses “have a closer relationship with SARS-CoV-2 than any other previously found in bats.”
HEROIC AS SUCH WILDLIFE surveillance may seem, some scientists question its value for heading off future pandemics.
PREDICT, which received $207 million from USAID from 2009 to 2019, discovered 959 novel viruses and identified hot spots for spillovers to humans, along with training Supaporn and nearly 7000 other researchers. “We were building their surveillance systems with them,” Mazet says.
Edward Holmes, an evolutionary biologist at the University of Sydney, applauds PREDICT’s training efforts but has doubts about whether the effort made the world safer. “It produced a fair amount of sequence data, but has it actually predicted anything?” he asks. “I don’t really know. It didn’t get SARS-CoV-2.”
Carroll, who retired from USAID in 2019, and scientists who participated in PREDICT contend that the project clarified what drives spillovers, such as the wildlife trade at markets and deforestation. PREDICT’s supporters also say it pinpointed sites where outbreaks are most likely. But Carroll readily acknowledges PREDICT’s limitations. “Its scope was too small to have a meaningful impact,” he says.
Hot zones
Researchers proposing a Global Virome Project have mapped regions where unknown viruses in wild mammals are most likely to spark human pandemics. Their predictions draw on data on known viruses, traits that predispose viruses to infecting humans, and human populations. Although Southeast Asia has been a hot spot of outbreaks driven by bat viruses, the Amazon region’s pandemic potential may be much higher.
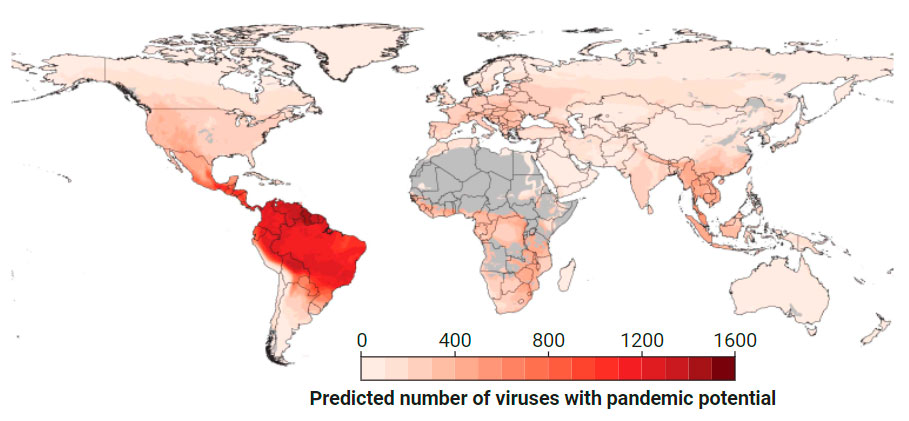
Carroll, Mazet, Daszak, and a small group of other researchers see PREDICT as a trial run for a much bigger effort: a GVP that aims to identify 75% of the viruses most likely to spill over within 10 years, at an estimated cost of $4 billion. GVP organizers, who started to flesh out the idea 6 years ago, had hoped to launch in 2020 with support from China and Thailand. The pandemic derailed their plans—but also underscored the need, Carroll, Supaporn, and other researchers argued last year in a commentary in The BMJ.
Holmes has assailed the idea of the GVP since it was first floated. “It’s absolute nonsense,” he says. “It’s too big a bloody arena.” Nearly all threatening pathogens are RNA viruses, which mutate at a fast clip, constantly creating new variants, Holmes notes. “You’ve got an amazing diversity of viruses that are continually turning over, so how would you then decide, ‘That’s the one that I’m worried about?’” he asks. “Surveillance is infinitely better and more cost-effectively directed at humans.”
Supaporn counters that the goal of wildlife surveillance isn’t to characterize every potential viral threat, but rather to learn how viruses evolve. And she is convinced that this work can predict the most likely future pathogens. “Even a general sense of this is extremely valuable to public health planning efforts,” she says. “Learn, understand, prepare.”
THOSE ARGUMENTS MAY HAVE contributed to Supaporn’s falling out with Thiravat, which forced her to walk away from the institution he heads, the Health Science Centre of the Thai Red Cross Emerging Infectious Diseases program at King Chulalongkorn Memorial Hospital. She is now at its sister Clinical Centre, without her equipment and trained technicians. Work like Supaporn’s promises more risks than benefits, Thiravat contends. “Wildlife surveillance may introduce human pathogens to wildlife and vice versa.” As for SARS-CoV-2, he believes it was not a natural jump of a virus from animals to humans. “It was a product of lab leak of virus after manipulation,” he asserts. (Thiravat has also advocated using the antiparasitic drug ivermectin to treat COVID-19, even though multiple studies have shown it is ineffective.)
Thiravat contends that Supaporn siphoned off about $400,000 from grants. But an investigation conducted by the Thai Red Cross Society exonerated her in July 2021, concluding in a letter (which she supplied to Science) that there was “no evidence of financial conduct contrary to [her employer’s] regulations.”
Some Supaporn supporters say Thiravat is jealous of the attention she has received for her coronavirus work during the pandemic. She says the problem began when she challenged things he said to his supervisors, which she did not want to discuss in detail. “I’ve always respected him—he is my mentor and an intelligent clinician and scientist,” she says. “And I’m lucky that even though I have some politics in the lab, people outside Thailand don’t think that I’m wrong, and they support me.”
Supaporn’s setbacks mean she must now rely on colleagues, including Wang, to complete the lab analyses of samples her team collects in the field. But she’s upbeat about her future.
In early March, she met with researchers from a new $125 million, 5-year project launched last year by USAID called Discovery & Exploration of Emerging Pathogens—Viral Zoonoses (DEEP VZN), taking them to the flying fox colony in the trees at Wat Luang Phrommawat. While she waits to see whether DEEP VZN makes her a collaborator and whether the GVP finds funding, Supaporn has enough grant money to continue her fieldwork for the time being.
For now, she focuses on training students and embracing the many unknowns she faces. “It’s a Buddhist teaching,” she says. “Uncertainty is certainty.”
Which could also be a motto for the entire pandemic prevention enterprise.
Fuente: https://www.science.org


